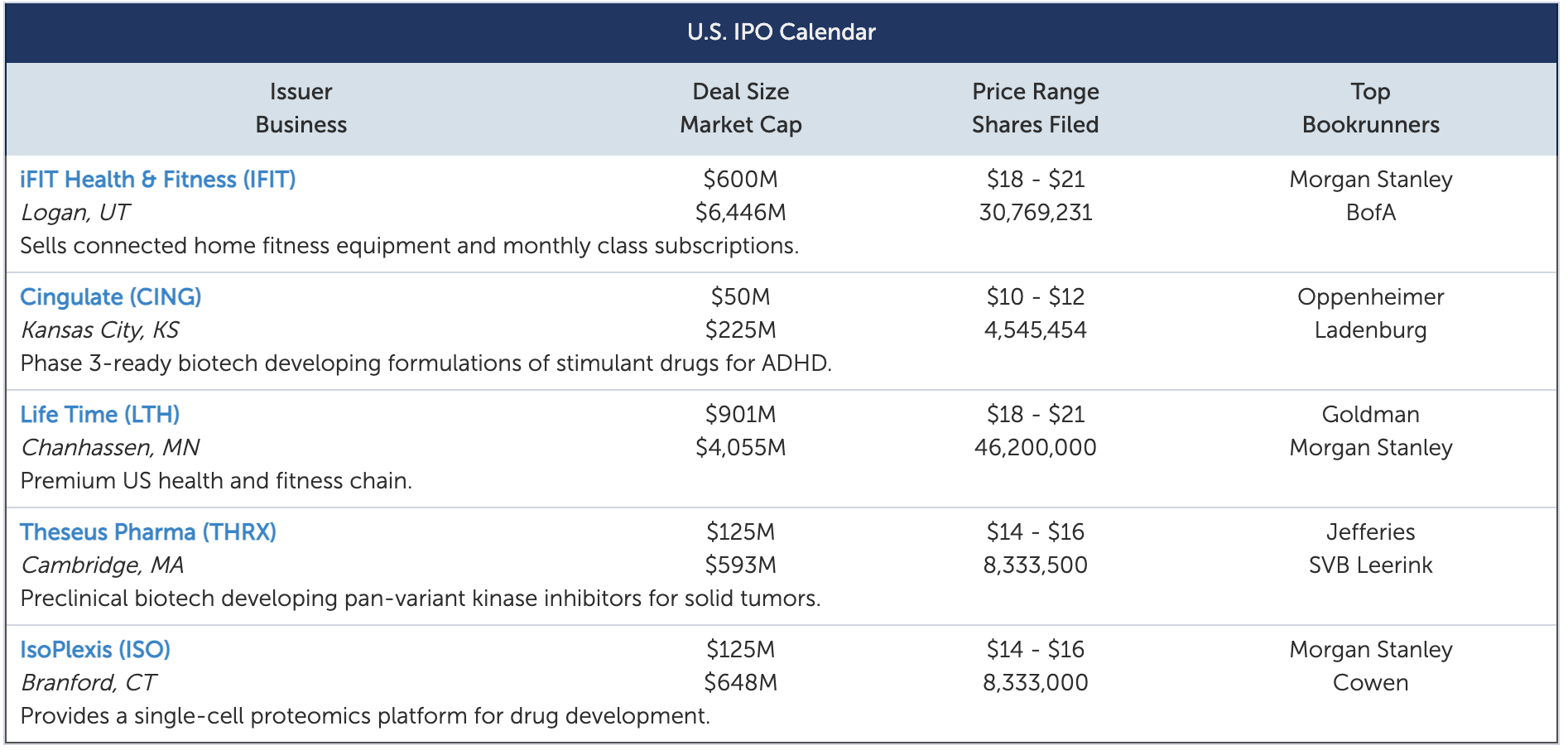
In the first full week of October, five IPOs are slated to raise $1.8 billion, led by two fitness companies.
Fitness chainLife Time Group Holdings(LTH) plans to raise $901 million at a $4.1 billion market cap. Taken private in 2015, Life Time operates more than 150 "centers" across 29 US states and one province in Canada, serving nearly 1.4 million individual members as of 7/31/21. While the company was hit hard by the pandemic, operations have since improved dramatically, with revenue quadrupling in the 2Q21.
Fitness equipment brandiFIT Health & Fitness(IFIT) plans to raise $600 million at a $6.4 billion market cap. iFIT is the #1 provider of large fitness equipment in the US, selling under brands including iFIT, NordicTrack, ProForm, and Freemotion. Fast growing and unprofitable, the company serves a community of over 6.1 million members and 1.5 million subscribers in over 120 countries.
Proteomics platformIsoPlexis(ISO) plans to raise $125 million at a $648 million market cap. IsoPlexis believes its platform is the first to employ both proteomics and single cell biology to characterize and link cellular function to patient outcomes. Fast growing and highly unprofitable, the company's platform has been adopted by the top 15 global biopharmas and nearly half of the comprehensive cancer centers in the US since its commercial launch in June 2018.
BiotechTheseus Pharmaceuticals(THRX) plans to raise $125 million at a $593 million market cap. Theseus’ lead candidate is a pan-variant inhibitor of all major classes of activating/resistance mutations of the KIT kinase for of gastrointestinal stromal tumors (GIST). The company recently submitted an IND for advanced GIST and plans to initiate a Phase 1/2 trial between late 4Q21 and mid 1Q22.
Drug developerCingulate(CING) plans to raise $50 million at a $225 million market cap. Its two candidates, CTx-1301 and CTx-1302, are being developed for the treatment of ADHD. The company announced positive results from a Phase 1/2 study of CTx-1301 in October 2020, and plans to initiate Phase 3 trials in the 4Q21 with results expected in late 2022.
精彩评论